
First authors: Thiago O. Machado, Connor J. Stubbs
Corresponding authors: Joshua C. Worch, Andrew P. Dove
Communication unit: University of Birmingham, UK
Paper doi: 10.1038/s41586-024-07399-9
Research background
The rapid manufacturing of customized 3D printed parts can be achieved by adding materials to photopolymerization resin through vacuum photopolymerization. Since the 1980s, advances in methodology have
continuously improved resolution and manufacturing speed, but process design and resin technology have largely remained consistent. A liquid resin formulation composed of reactive monomers and/or oligomers
containing (methyl) acrylate and epoxides, rapidly polymerizes under light stimulation in the presence of a photoinitiator to form a cross-linked polymer network. Most of these resin components are obtained from
petroleum raw materials, although recent progress has been made through the derivatization of renewable biomass and the introduction of hydrolyzable bonds. However, the obtained materials are still similar to
traditional cross-linked rubber and thermosetting resins, which limits the recyclability of printed materials. At present, there is no existing photopolymer resin that can depolymerize and be directly reused in a cyclic and
closed-loop manner.
Highlights of this article
1. This work describes a photopolymer resin platform derived entirely from renewable fatty acid salts, which can be 3D printed into high-resolution parts, effectively deconstructed, and then reprinted in a cyclic manner.
2. The inefficiency of the previous method of using internal dynamic covalent bonds to recover and transfer 3D printed photopolymers was solved by replacing traditional (methyl) acrylate with dynamic cyclic disulfide
species in lipoic acid s.
3. The lipoic acid resin platform is highly modular, and its composition and network structure can be adjusted to obtain printing materials with different thermal and mechanical properties, which can be comparable to
several commercial acrylic resins.
Image and text analysis:
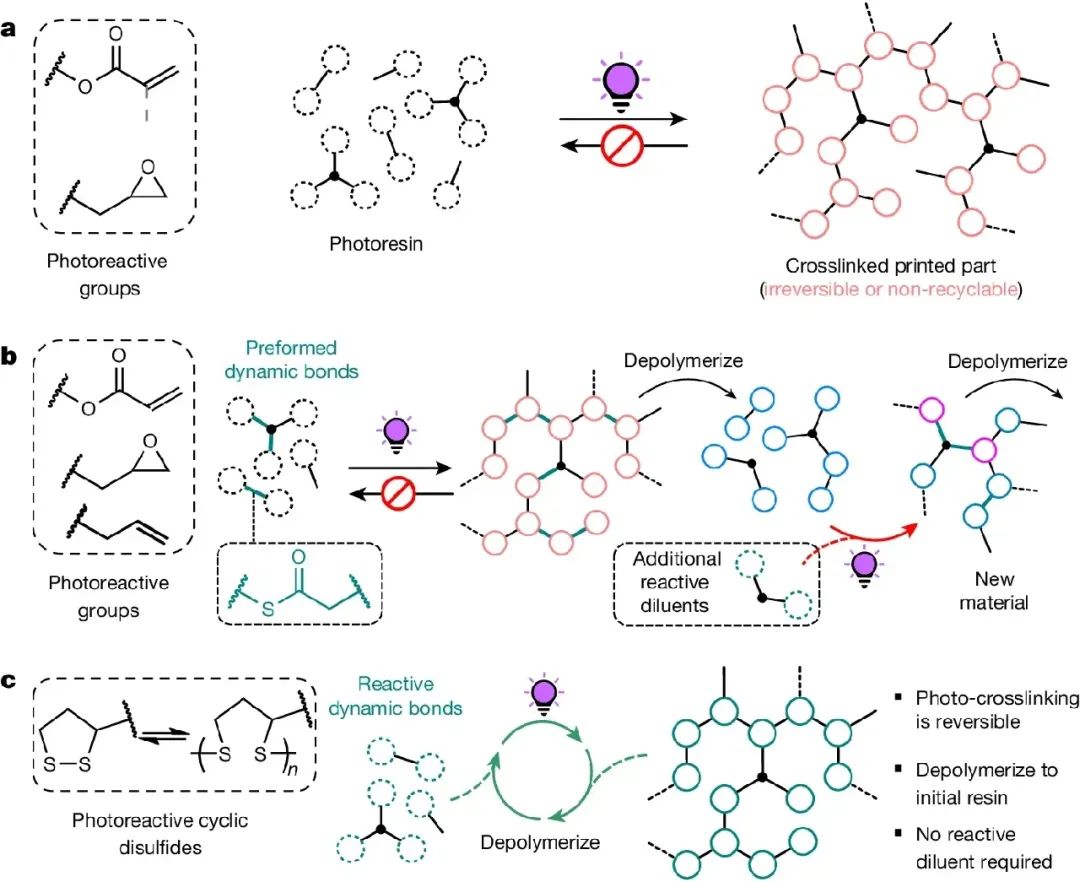
Figure 1. 3D printing and recycling of UV cured resin
Key points:
1. This work describes a photopolymer resin platform derived entirely from renewable fatty acid salts, which can be 3D printed into high-resolution parts, effectively deconstructed, and then reprinted in a cyclic manner.
The most advanced photocuring dynamic network can be redesigned for reprinting or reproduction by adding additional photoactive resin components during the ring opening process. However, the orthogonality
between dynamic bonds and photochemical crosslinking reactions results in the regenerated material having a unique composition (compared to the original sample).
2. In addition, additional active species are required in each continuous recycling step, which leads to the "snowball" effect, in which the only way is to make the material more abundant. To achieve a circular
photopolymerization network, dynamic bonds must be formed through photopolymerization, which is formed in situ during the crosslinking process. The network must be depolymerized back to its original units for
repeated photopolymerization or printing (Figure 1c).
3. Due to their ability to polymerize through free radical mediated methods and establish dynamic behaviors including depolymerization, this work proposes that using strain cyclic disulfides, such as naturally sourced
lipoic acid, can allow for rapid curing by maintaining a sufficiently high concentration of disulfides in the resin without the need for additives, which will make the material irreversible but not destabilize the resin, thus
achieving closed-loop vat photopolymerization printing (Figure 1c).
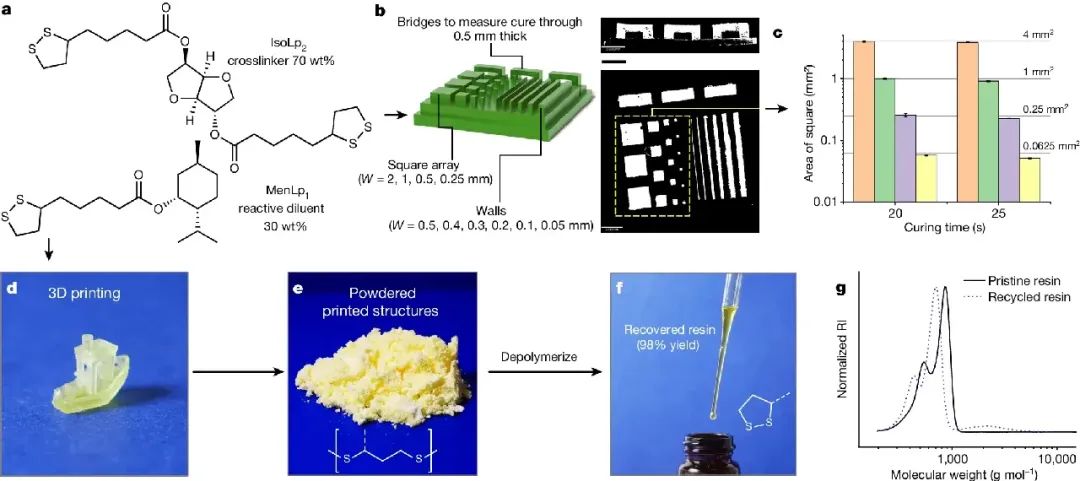
Figure 2. 3D printing of MenLp1 IsoLp2 (30:70 wt%) and depolymerization of printed parts
Key points:
1. When diluted in solvent, both types of lipoic acid s exhibit good environmental stability; However, in terms of concentration, IsoLp2 is prone to gel during long-term storage, which may be due to premature self
crosslinking. However, when two components of lipoic acid were mixed to form resin Men Lp1 Iso Lp2 (30:70 wt%), we observed that the mixture was more stable than any individual component (Figure 2a), which may be
due to each mixed component acting as a diluent for the other component, although also having cyclic disulfide bonds.
2. In order to evaluate printing resolution and fidelity, this work designed a rectangular substrate containing several square arrays and bridges, inspired by the method of Page and colleagues (Figure 2b). The minimum
feature that can be reprinted in this job is 100 μ M walls (approximately 3 pixels wide), every 50 μ M requires 25 seconds, which highlights the impressive x-y resolution of the resin platform in this work on ready-made
commercial 3D printers.
3. In addition, the successful printing of the bridge structure demonstrates good resolution along the z-axis direction, with only moderate penetration of the suspended bridge observed in the z-axis direction (113 ± 7%
at 20 s curing) (Figure 2b). Commercial acrylic resins typically incorporate a sunshade to suppress curing that distorts the z-resolution (over curing greater than or equal to 200-300%) without the addition of a sunshade.
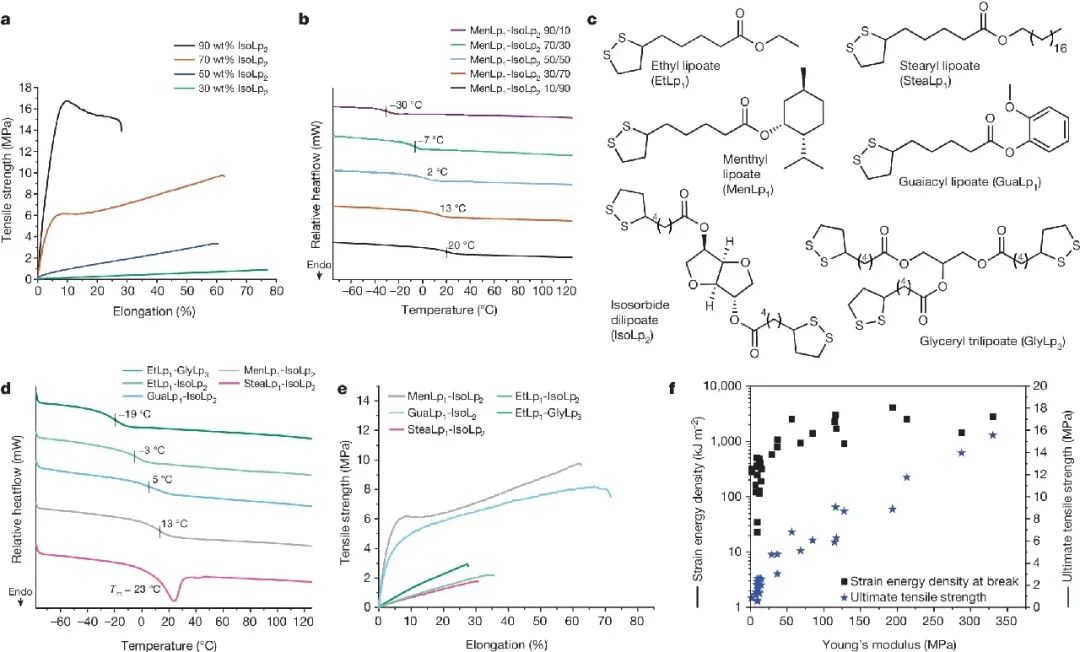
Figure 3. Thermal and mechanical properties of post cured 2D photocurable materials obtained from several renewable lipoic acid resins
Key points:
1. In order to explore the structure performance space of the platform, this work first changed the ratio of active diluent to crosslinking agent in the MenLp1-IsoLp2 (90:10 to 10:90 wt%) formula (Figure 3a, b).
Each residual solution is stored at room temperature without the need for additional diluents. This work produced a 2D photo set with a thickness of approximately 0.5 mm by curing it on a glass slide (approximately
300-500 nm, irradiated for 30 minutes, (1) wt% ethyl (2,4,6-trimethylbenzoyl)) phenylphosphinate, to achieve rapid material screening.
2. By utilizing other renewable sources of alcohols to synthesize a series of monomer fatty acid esters, this work can explore the universality of resin platforms. By combining Iso Lp2 with RLp1 in a ratio of 30:70wt%
(diluent to crosslinking agent) and subsequent UV curing formulations, this work created a material with a range of mechanical properties (Figure 3c-f).
3. The current efforts to further improve mechanical properties in this work are focused on combining the inherent flexibility of low glass transition temperature with network enhancement through non covalent bonding
interactions, such as metal coordination strategies, stereochemical effects, or hydrogen bonding groups. The use of hydrazide introduces strong hydrogen bonding interactions, resulting in a network of lipoic acid with a
UTS close to 50 MPa and a Young's modulus as high as 340 MPa, proving that the substantial hardening of the lipoic acid resin platform can achieve wider competition with state-of-the-art commercial resins.
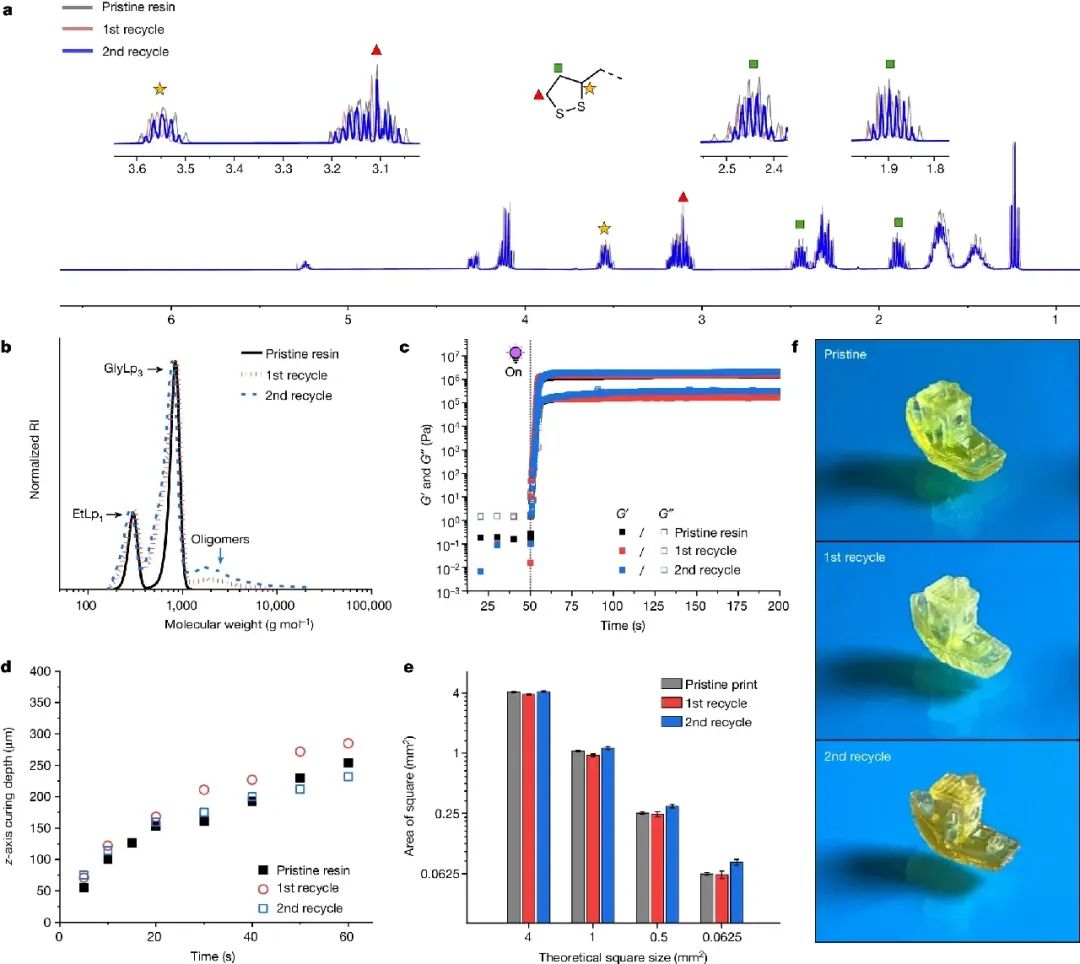
Figure 4. Cyclic DLP printing of EtLp1-GlyLp3 (31:69 wt%) resin
Key points:
1. The efficient depolymerization of the cured EtLp1-GlyLp3 material and the overall high recovery rate of the recycled resin allowed us to conduct a more in-depth study on it in a closed-loop 3D printing cycle, during
which our work successfully completed two cycle sequences. Successfully printed EtLp1-GlyLp3 (31:69 wt%) resin, and then efficiently depolymerized it into a high yield (91%; 94%) using a thermal assisted
depolymerization method in DMF. The composition of the original resin and the regenerated resin is comparable, as determined by 1H NMR.
2. The SEC analysis of the three resins showed that they have similar molecular weights, although a small amount of oligomers (molecular weight greater than 1000 g mol-1) were observed in the recovered products
(Figure 4b), which is consistent with the 1H NMR spectrum data. Ultraviolet visible spectroscopy (UV vis) analysis also showed slight differences in absorption profiles, and the recovered resin was λ= The shoulder peak
appears near 290 nm, which we also attribute to the smaller oligomer impurities.
3. These results demonstrate the conceptual progress of cyclic DLP printing in the field of photocurable resin additive manufacturing. The use of renewable, sustainable, and harmless fatty acid esters solves these
limitations of state-of-the-art resins and has broader application prospects. The current efforts are focused on improving the orthogonality of network depolymerization, i.e. eliminating the presence of oligomeric
pollutants to reduce minor differences in resin composition in scale recovery.






